You’ve probably heard the term water hardness and wondered what it’s all about.
Is it bad or is it good?
Where does it come from?
Well, to give you a better understanding, we have prepared this detailed article to explain almost everything you need to know about water hardness and hard water.
We will look at what it is, what makes it, the different levels of water hardness as well as the difference between the two main types of water, their pros, and cons, what to know about hard water treatment, and much more.
Water Hardness
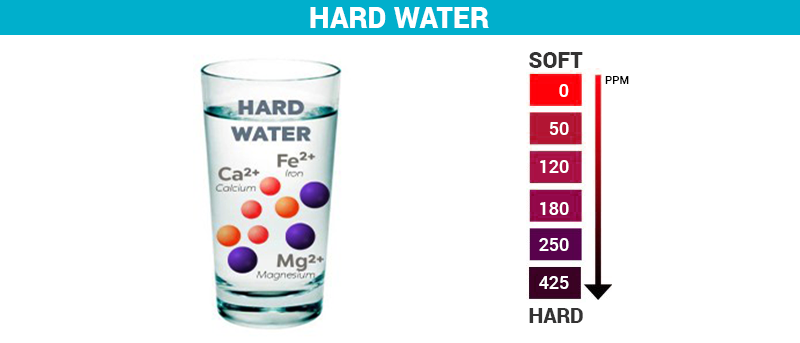
Water hardness refers to the total concentration of divalent metal cations in water. Divalent cations are positively charged metal ions with a charge of 2+. Dissolved calcium ions (Ca2+) and magnesium ions (Mg2+) are the two principal divalent cations that create water hardness.
In natural water, calcium and magnesium minerals primarily exist as dissolved compounds (salts). It’s the amount of the dissolved salts of these two minerals in water that causes hardness. Based on the hardness, we have two main types of water; hard water and soft water.
What makes water hardness
As mentioned above, the principal causes of water hardness are calcium and magnesium minerals. These minerals occur naturally as ionic salts (primarily as bicarbonates, sulfates, nitrates, and chlorides) in sedimentary rocks such as limestone, chalk, dolomite, and gypsum.
As surface water (streams and rivers) or rainwater flows and percolates through these rocks, it dissolves the calcium and magnesium mineral salts in them which causes hardness.
Calcium, in particular, is dissolved in water as it flows over and through limestone deposits and chalk (which are mainly calcium carbonate) or other calcium-bearing formations like gypsum (calcium sulfate).
Magnesium, on the other hand, is dissolved as the water flows over and through dolomite (calcium magnesium carbonate) or other magnesium bearing formations like magnesium sulfate from salt deposits underground.
Because groundwater is often in contact with these rocks for a longer period than surface water, it usually has a high degree of hardness than surface water. In other words, the more calcium and magnesium mineral salts dissolved in the water, the higher the degree of hardness.
Hardness in groundwater can also occur from chemicals and mining industry effluent as well as from excessive application of lime to the soil in agricultural areas.
A minor contribution to the hardness of water is also made by polyvalent ions like strontium, aluminum, iron, barium, manganese, and zinc.
Types of Water Hardness
Temporary hardness
Temporary hardness, also known as ‘carbonate hardness’, is the total hardness of water that can be removed by boiling water or the addition of lime (calcium hydroxide).
What causes temporary hardness in water?
Temporary hardness is caused by the presence of bicarbonate (otherwise known as, hydrogen carbonate, HCO3–) minerals in the water, mainly calcium bicarbonate and magnesium bicarbonate.
Insoluble calcium carbonate (in chalk and limestone) and insoluble magnesium carbonate become dissolved in naturally carbonated water like acid rainwater (naturally acidified with carbon dioxide gas which is dissolved from the atmosphere) to form soluble bicarbonates.
- CaCO3(s) + H2O(l) + CO2(g) è Ca(HCO3)2(aq)
- MgCO3(s) + H2O(l) + CO2(g) è Mg(HCO3)2(aq)
The H2O + CO2 is also written as H2CO3 which is referred to as ‘carbonic acid’ or naturally carbonated water. The dissolved carbon dioxide gas makes rainwater slightly acidic so it’s able to react with the carbonate and slowly dissolve limestone and chalk.
The bicarbonates (or hydrogen carbonates) of calcium and magnesium that cause the temporary hardness in water are thermally unstable hence decompose on heating. This is why temporary hardness is removable by boiling the water.
The soluble calcium bicarbonate and magnesium bicarbonate decompose into insoluble calcium carbonate and magnesium carbonate, and precipitate, leaving softer water and expelling CO2 in the process.
- Ca(HCO3)2 ⇋ CaCO3 + CO2 + H2O
- Mg(HCO3)2 ⇋ MgCO3 + CO2 + H2O
Permanent hardness
Permanent hardness, also known as ‘non-carbonate hardness’ is water hardness that can’t be removed through boiling.
What causes temporary hardness in water?
Permanent hardness in water usually occurs due to the presence of very soluble magnesium sulfate (dissolved from salt deposits underground)/magnesium chloride, and the slightly soluble calcium sulfate (dissolved from gypsum deposits)/calcium chloride.
Iron, aluminum, manganese, and several other heavy metals present in water can also cause permanent hardness but they are usually not in large concentrations in natural water to contribute significantly to total permanent hardness.
The sulfate and chloride salts of magnesium and calcium cause permanent hardness in water because they are unaffected by heat. They don’t precipitate out as the temperature rises, therefore boiling the water doesn’t remove the hardness.
Water Hardness Measurement
Water hardness is expressed in various units but the most commonly used units are grains per gallon (gpg), milligrams per liter (mg/L), and parts per million (ppm).
Parts per million (ppm) is basically a weight-to-weight ratio which means 1 ppm of calcium translates to one pound of calcium in one million pounds of water (or one gram of calcium in one million grams of water).
Grains per gallon (gpg) is a weight measurement that typically refers to the weight of a substance in 1 gallon of water. It’s the most commonly used measurement for expressing water hardness. 1 grain is equivalent to 64.799 milligrams while 1 gpg is equivalent to 17.14 ppm.
Milligrams per liter (mg/L) is similar to ppm. It’s a measure of the concentration of a substance by weight per unit volume in water. 1 gpg is equivalent to 17.14 mg/L.
Since calcium carbonate is amongst the most common causes of hardness, the total hardness of water is usually expressed based on the calcium carbonate concentration (mg/L or ppm as CaCO3).
The reason behind the use of calcium carbonate concentration is that when a certain mass of calcium carbonate dissolves in a unit volume of water, it would result in an equal total molar concentration of Ca2+ and Mg2+. In other words, water hardness is measured as if all the magnesium and calcium in water were present only as calcium carbonate.
Water Hardness Levels (Water Hardness Classification)
Water hardness levels refer to the degree of hardness of water with regard to its calcium carbonate concentration.
Most municipal water is drawn from local sources like surface water and groundwater. As such, variations in the degree of water hardness can occur depending on the source as well as its location.
For instance, water from glaciers, streams, and rivers flowing through and over igneous or sedimentary rocks is likely to have a low degree of hardness since it’s only in contact with these rocks for a short period hence doesn’t dissolve more limestone.
On the other hand, groundwater-like water obtained from boreholes that are drilled into porous rocks usually has a high degree of hardness. The hardness of groundwater can also vary depending on the depth from which it’s drawn.
For instance, water obtained from shallow aquifers may have a slightly low degree of hardness especially during a period of heavy rain. This is because the aquifers fill up and as a result, dilute the hardness. Water in deep aquifers (at depth of over 500 feet) may have a high degree of hardness since it’s in the aquifer for longer hence capable of dissolving more limestone.
Water hardness scale table
| Grains/Gal | Mg/L & PPM | Classification |
|---|---|---|
| Less than 1 | Less than 17.1 | Soft |
| 1 – 3.5 | 17.1 – 60 | Slightly hard |
| 3.5 – 7 | 60 – 120 | Moderately hard |
| 7 – 10 | 120 – 180 | Hard |
| Over 10 | Over 180 | Very Hard |
Water Hardness Scale Chart

Water Hardness vs. pH Of The Water
While water hardness and pH are different properties of water, the two are closely related. pH determines how acidic or basic water is, hardness measures the number of dissolved minerals (calcium and magnesium) in the water.
The reason why the two are closely linked is that dissolved minerals tend to combat the effects of acids in water (a process that is otherwise known as buffering), thereby preventing the pH from dropping.
Due to this, hard water (which has high mineral content) usually has a high pH making it more alkaline. The high mineral content acts as a buffer hence reducing the amount of acid in the hard water. Likewise, soft water tends to have low mineral content hence it’s usually low in pH which makes it more acidic.
Know Your Water Hardness
Water hardness report by local area/city/zip code
Determining water hardness can be a daunting task. However, you can get a much more precise measurement of the water hardness level within your local area from your water provider or by entering your ZIP code or local water utility’s name online.
For example, in the U.S, the Environmental Working Group (EWG) has an online Tap Water Database that contains results from tests done in all 50 states by state agencies like the Environmental Protection Agency (EPA).
It’s a searchable database that allows you to enter your ZIP code or local water utility name to find out what’s in your tap water. You can also request official reports of water hardness in your city or local area.
Water hardness map
A water hardness map is a useful tool and guide that can also help you determine the water hardness in your respective local area. This too can be accessed online via government agencies’ websites like the United States Geological Survey.
The water hardness map can help you find your city’s specific water hardness measurement based on reports from both government and private sources.
Water hardness testing
Using a Tester
Another option that can give you quick results is testing your water yourself. You can easily do this using an at-home water hardness test kit which can be purchased from your local hardware store or online.
A typical water hardness testing kit consists of a color chart and paper test strips. These are very easy to use. You just fill a glass with water from your tap and dip a test strip for a few seconds and then remove it.
The strip will change t color upon which you compare it against the color chart. Each color on the chart represents a particular hardness level (measured in gpg, ppm, or mg/L), and based on the color of your strip, it should tell you the hardness of your water.
Testing services
Your local water provider may as well be able to tell you the precise hardness level of your water supply. You can contact them and request the results of their water tests to know your water hardness.
In case you have a private well or just want your own individual water tested, then you can contact your local health department and schedule an appointment for testing.
The other option is having a private company perform an on-site test. Several water softener companies may offer a free water test in the hope that you’ll use their services. In this case, they may send a specialist to your home to perform the water hardness test or request a sample of your water and send back the results.
You can also send a sample of your water to any professional water testing lab within your local area where they will conduct tests to determine its hardness.
Hard Water
Hard water is water that contains high mineral content (mainly calcium and magnesium) absorbed from the soil and rocks such as limestone, chalk, and dolomite.
The concentration of calcium and magnesium minerals determines how hard it is. Therefore, so water that contains large amounts of dissolved calcium and magnesium is considered very hard.
The Advantages
Safe To Drink and Provides Essential Minerals
According to World Health Organization (WHO), hard water has no known serious health effects. It’s safe for drinking and moreover, it could be a supplementary contributor to the total calcium and magnesium intake.
The World Health Organization (WHO) says that due to the high concentrations of calcium and magnesium in hard water, drinking it may help one meet the recommended daily intake of these important minerals, especially for those who have marginal calcium and magnesium intake in their diet.
Besides that, hard water doesn’t dissolve lead. As such, it doesn’t result in lead poisoning in case lead pipes are used for supplying water into households.
May Lower Cardiovascular Disease Mortality
Several large-scale studies have reported an inverse link between hard water and cardiovascular disease. Earlier studies have found a positive correlation between water and dietary calcium and magnesium and blood pressure.
Studies carried out in South African and Finland found that the cause of death attributed to ischemic heart disease (coronary heart disease, CHD) is inversely correlated with the amount of magnesium in drinking water.
Another study of magnesium in drinking water and mortality due to acute myocardial infarction carried out in North-West England stated that there could be a possible association between magnesium in hard water and cardiovascular mortality.
Other studies show that there’s a substantial protective effect of magnesium consumption from hard water on the risks related to cerebrovascular disease.
Generally, although there are no definitive conclusions that back up most of these claims, the few studies carried out do link hard water consumption to lower cardiovascular mortality.
Strengthens Bone and Teeth
Drinking hard water can be beneficial too in strengthening our bones and teeth. The reason for this is that our teeth and bones are mainly built from calcium.
Therefore, hard water is capable of making them stronger because it tends to contain high amounts of calcium as well as a significant amount of iron, both of which are good for the teeth and bones.
May be Beneficial to Digestive Health and Constipation
The right combination of calcium and manganese can reduce constipation and as well help to fight it. Furthermore, calcium which is present in hard water in high amounts combines with the excess fats and bile which are then eliminated from the body by bowel movements.
This means, that drinking hard water may help with constipation and even possibly improve your digestive health. Additionally, a lot of people may consider hard water as it has a more pleasant taste compared to soft water which tends to be tasteless.
The Disadvantages
Can Cause Dry Skin and Hair
While there are no adverse health problems associated with drinking hard water, its effect on hair and skin is a different story. Washing your hair frequently using hard water can cause it to become dry and rough.
The reason for this is that the excess minerals in the hard water tend to form a curd-like substance which sticks to your hair or leaves behind a film.
As a result, it reduces moisture and makes the hair frizzier. This may also leave your scalp feeling itchy and can as well cause dandruff.
Taking a bath or shower with hard water can make your skin itchy and dry too. This is because it leaves behind a soap residue that sticks to your skin. The leftover residue reduces moisture which can instigate eczema-like symptoms. This is an issue that’s more common in children.
Moreover, the minerals in hard water may change the skin’s pH balance, thereby weakening it as a key barrier against infections and harmful bacteria. Those with eczema may be more vulnerable.
Can Cause Havoc on Appliances
Hard water often leaves scale deposits on appliances which can damage them or make them work harder and also use more energy.
For instance, when hard water is heated in a home water heater, it forms solid deposits of calcium carbonate. This scale deposit can lower the efficiency of the water heater and cause clogging thereby raising the costs of heating water.
It can ultimately reduce the lifespan of appliances. A good example is a washing machine which has an expected life span of 11 years but if you operate it using hard water, then the lifespan tends to reduce to around 7 years.
Scale buildup resulting from hard water can also occur in your home appliances like the coffee maker, dishwasher, and geysers, thereby reducing their efficiency and life span. You may also see scale build-up on your showerhead and sink faucets.
Industries located in areas where water is relatively hard might as well have to spend a significant amount of money to soften their water since the hard water can damage their equipment.
Can Cause Clogging in Water Pipe Systems
The long-term flow of hard water through pipes can also lead to scale build-up which reduces their inside diameter. As a result, the water pipes may gradually close up causing less water flows through the pipes as well as a lowering of water pressure. The scale build-up can eventually clog the pipes completely resulting in unwanted costs of having to replace them.
Can Shorten the Life of Clothes and Fabrics
Washing clothes using hard water may cause the fabric to break down quickly over time due to the residue or “soap scum” (formed when calcium in hard water reacts with the soap) left in the fabric. The residue also often causes the clothes to fade and have a dingy appearance.
This is the same case with dishes and glasses when using hard water in dishwashers. They usually come out with spots and residue. Additionally, hard water doesn’t lather easily, and as such, more soap and detergent are required to get things clean, especially when washing clothes.
Soft Water
Soft water is water that contains low concentrations of dissolved minerals. In particular, it’s low in ions of magnesium and calcium. Water that contains less than 120 mg/L can also be classified as soft based on the World Health Organization guidelines.
Snow, rainwater, and water from glaciers are some good examples of naturally soft water since they have little contact with rocks and soils bearing calcium and magnesium content. It can also occur naturally where rainfall and river basins are formed.
The Advantages
Long-Lasting Appliances and Plumbing
Hard water has a high concentration of calcium and magnesium minerals that cause a lot of scale build-up over time which destroys the plumbing system. The clogs in pipes not only affect the movement and pressure of water but can also lead to leakages or even burst pipes.
Conversely, since soft water has a very low mineral content, it’s far gentler hence putting significantly less strain on the plumbing system. By using soft water, you get to protect your plumbing fixtures and as well easily increase the lifespan of your entire plumbing system.
Soft water can also save you a great deal in terms of appliance replacement costs and repairs. It can save you from costly energy bills too because there is no lime build-up to reduce the efficiency and performance of your appliances, especially water heaters and washing machines.
Leads to Softer, Moisturized Skin and Hair
Hard water is well-known for drying out skin and hair as well as causing itchiness. It leaves soap residue on the skin that potentially clogs the pores and also the minerals in it tend to remove the natural oils from the skin causing it to become dry and itchy.
However, showering or bathing in soft water can reverse these effects because there’s no soap or mineral residue that’s left on the skin, scalp, or hair.
It makes the skin feel softer and more moisturized as the pores are not blocked by residue and the natural oils are not wiped away like in the case of hard water, plus it can help revitalize dull, lifeless hair. It’s especially useful when shaving too since it allows razors to move more smoothly across the skin.
Cleans More Efficiently
Soft water is an efficient and effective cleaning agent compared to hard water. The soap lathers up far much better with soft water and rinses off easily leaving clothes and dishes cleaner.
This ability to produce lather from soap much more efficiently is what makes it very effective at cleaning and rinsing. The best part is that, unlike hard water, soft water doesn’t form soap scum or mineral stains hence clothes and fabrics not only become cleaner but also don’t fade quickly.
Another bonus is that you get to use much less soap when washing clothes using soft water because the soap lathers up easily hence saving you some money you would spend on the regular purchase of soap and laundry detergent. It can also save you some money on your water bill since you won’t have to re-wash clothes or dishes every time.
The Disadvantages
Devoid of Essential Mineral Content
The main disadvantage of soft water is the fact that it has lower concentrations of essential minerals which are important to our health.
Furthermore, although it’s safe to drink, artificially softened water can be totally devoid of essential minerals thereby it may not offer many health benefits especially if there are deficiencies of essential minerals in your diet.
However, if your diet includes foods rich in calcium and magnesium content like fruits and vegetables, then there’s likely no risk in drinking soft water that has lower concentrations of these minerals.
May Not be Ideal if You are Sensitive to Heightened Sodium Levels
According to a report from the University of Kentucky, drinking soft water regularly can be harmful to individuals who are sensitive to heightened sodium levels (high salinity). These include diabetics and those with high blood pressure.
The reason for this is because sodium levels are usually much higher in soft water, particularly, water that has been chemically softened or gone through a softening process known as ion exchange.
Calcium and magnesium ions are replaced with sodium ions during the ion exchange process which increases the sodium levels in the softened water. So, if you need to limit your sodium intake, then softened water may not be the best choice.
Can Leach Heavy Metals from Pipes and Faucets
There’s evidence that softened water may be more volatile and as such, it has the potential to pick up heavy metals like lead, cadmium, copper, and zinc from pipes and plumbing fixtures.
The concentrations of these heavy metals can exceed the primary drinking water standards specified by the Environmental Protection Agency (EPA), particularly for softened water that’s left standing for long in plumbing fixtures and faucets made of brass, which could potentially be detrimental to health.
Hard Water Treatment
Water Softening (Ion exchange)
Water softening is the process of removing hardness from water. It’s a technique that enables the removal of calcium and magnesium ions which are what cause water to be hard.
One of the best ways of softening water is using a water softener which is a specific ion exchanger that’s designed to remove positively charged ions.
Water softeners mainly remove the calcium (Ca2+) and magnesium (Mg2+) ions present in the water by replacing them with other ions such as sodium or potassium (also known as exchanger ions).
These exchanger ions are added to an ion exchanger (water softener) reservoir as either sodium or potassium salts (NaCl and KCl). The hardness minerals are collected within its conditioning tank where they are flushed away to drain from time to time.
Why (which cases) you may need to soften your water:
You may need to soften your water if you notice the following signs of hard water:
- You notice a hard, chalky film (scale buildup) on your appliances (like washing machine, dishwasher) and plumbing fixtures (like showerheads and faucets).
- Your plumbing system requires constant repairs or water faucets start to have low flow.
- You see rusty looking stains on bathtubs, sinks, toilets and showers. Water spots on kitchenware like dishes, pots, glassware and silverware are also signs of hard water.
- Noticing that your clothes seem faded or gray is an indication of hard water.
- Your skin often feels itchy and dry after showering, or your hair seems dingy or dull.
- You notice your water bill has recently skyrocketed.
For preventing limescale buildup purpose only.
Besides water softeners (ion exchangers), there are two alternative solutions that you can also use to deal with hard water problems. This includes using either a water conditioner or a water descaler.
Unlike water softeners, these two solutions which are also known as “salt-free water softeners” do not actually remove the hardness minerals (calcium and magnesium), instead, they help prevent limescale build-up on pipes and appliances.
Water Conditioner
A water conditioner is a device that utilizes a catalytic media (placed in a tank or cartridge) to transform the hardness minerals into harmless crystals using a physical process known as Template Assisted Crystallization (TAC).
The crystals formed are stable and do not adhere to the surfaces that the water comes into contact with thereby eliminating the problem of limescale buildup. Note that this system does not soften the water, it simply conditions it.
Water Descaler
A water descaler is an electronic device that’s made up of coils of electrical cables which are wrapped around the pipes within the main water inlet. They utilize electromagnetic waves to prevent limescale build-up by altering the chemical structure of the hardness minerals. The minerals remain in the water but are unable to stick inside pipes and plumbing fixtures.
Why (which cases) you may need to use an alternative solution
- You have limited space – a salt-free water softener like the water descaler is very compact in terms of physical design hence allows you to save on space plus you can install it practically anywhere inside or outside, provided it’s protected from freezing.
- You are sensitive to high sodium levels – if you have cardiovascular problems or you are trying to limit your sodium intake, then a water descaler or water conditioner might be ideal for you since unlike water softeners, these won’t add sodium to your water.
- You don’t want to remove the essential minerals – unlike water softeners, water descalers and water conditioners only prevent limescale build-up. They don’t really remove the essential minerals in the water hence they are ideal if you want to keep the mineral content of your water.
- You want to conserve water and save some money – salt-based water softeners (ion exchangers) require electricity, salt and other chemicals to soften water. They also use a lot of water since they generate wastewater which salt-free softeners don’t which makes them a cheaper option when it comes to the running costs.
Conclusion
As you’ve seen from this compressive article, water isn’t simply water as many people would think. Despite the potential health benefits that come from drinking hard water, the hardness can become a nuisance for the most part.
If you reside in an area where water hardness levels are too high, you don’t have to put up with the negative impacts of hard water in your home.
You can protect your plumbing systems appliances and even skin and hair by softening your water using the available solutions we’ve mentioned above. The alternative solutions, in particular, are ideal for dealing with mild water hardness.
However, if you want a more complete and thorough solution to treating high water hardness levels (above 3 GPG), then a whole-house water softener system would be the best choice.
Table Of Content
- What is water hardness
- What makes water hardness
- Types of Water Hardness
- Water Hardness Measurement
- Water Hardness Levels
- Water Hardness vs. pH Of The Water
- Know Your Water Hardness
- Hard Water
- Soft Water
- Hard Water Treatment
- Conclusion
Reference sources
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/water-hardness
- https://www.usgs.gov/special-topic/water-science-school/science/hardness-water?qt-science_center_objects=0#qt-science_center_objects
- http://www.docbrown.info/page01/AqueousChem/AqueousChem3.htm
- http://www.ewg.org/tapwater/
- https://www.usgs.gov/media/images/quality-water-domestic-wells-united-states
- https://www.livescience.com/59935-tap-water-database.html
- https://www.infrastruktura-bled.si/en/Services/Water-supply/WATER-HARDNESS
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3775162/#ref3
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1601548/
- https://www.wqa.org/learn-about-water/perceptible-issues/scale-deposits
- https://www.eastorange-nj.gov/361/Softening-Your-Water

Nicely done article….thank you.
Does an abundance of snow or rain into the reservoirs periodically decrease the water’s hardness?
Thanks John for your comment. It depends on how the rainwater or snow comes to the reservoirs. The water hardness can be decreased if the rainwater comes directly into the reservoirs, otherwise, the water may contain more dissolved calcium ions that make the water harder.
Great explanation! In Phoenix, we have 18 gpg water and PEX plumbing. I want to replace the water softener and would greatly appreciate your suggestions.
I’m thinking of installing an electromagnetic coil on the incoming “soft water loop” followed by a TAC filter. Do you think that would work? Do you have suggestions regarding the best products?
Thanks!